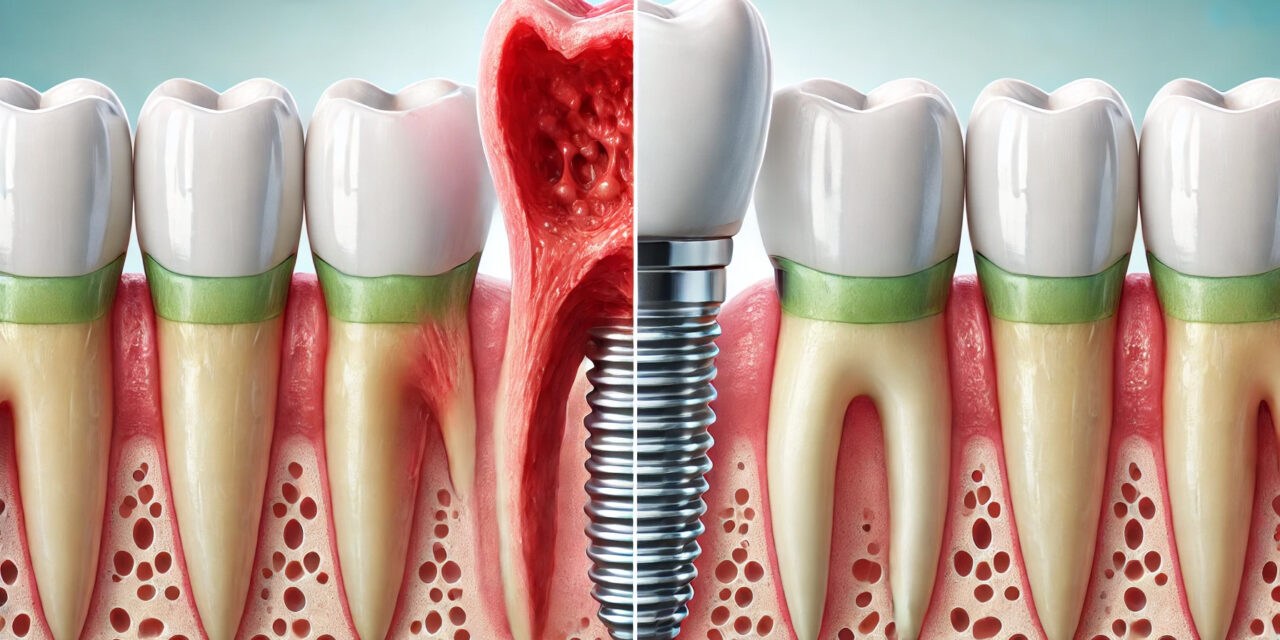
Peri‑implantitis has emerged as one of the most pressing challenges in modern implant dentistry, threatening the longevity of otherwise successful restorative work. Characterized by inflammation and progressive bone loss around osseointegrated implants, this condition demands early detection and evidence‑based intervention to preserve implant function and patient trust.
Understanding the Pathogenesis
Peri‑implantitis develops from a complex interplay of microbial, host, and mechanical factors. Biofilm accumulation is the primary driver, with gram‑negative anaerobic bacteria such as Porphyromonas gingivalis and Tannerella forsythia playing a major role¹. Unlike natural teeth, implants lack the same connective tissue fiber arrangement, making peri‑implant tissues more susceptible to rapid inflammatory spread².
Other contributing mechanisms include:
- Microgap leakage at the implant–abutment interface facilitating bacterial ingress.
- Excess cement around abutments acting as a nidus for inflammation.
- Occlusal overload accelerating marginal bone loss.
Key Risk Factors to Watch
A strong body of research highlights both local and systemic factors that increase susceptibility:
- History of periodontitis – Strongly associated with higher peri‑implantitis incidence³.
- Poor plaque control – Consistently linked to disease initiation and progression⁴.
- Smoking – Impairs immune response and microcirculation⁵.
- Diabetes mellitus – Particularly uncontrolled cases affect healing and inflammatory regulation⁶.
- Inadequate maintenance care – Lack of routine supportive therapy increases risk⁷.
Patients presenting with multiple risk factors require stricter maintenance schedules and more rigorous monitoring.
Diagnosis: Catching It Early
Early diagnosis is the single most important factor in preserving implant health. The challenge lies in differentiating peri‑implant mucositis — a reversible inflammatory reaction of the peri‑implant soft tissue — from peri‑implantitis, which involves irreversible bone loss.
Key Clinical Indicators
- Bleeding on probing (BOP): Persistent BOP is a hallmark of early disease. Even in the absence of suppuration, it warrants closer monitoring.
- Increased probing depth: Pocket depths greater than 5 mm compared with baseline should raise concern.
- Suppuration: The presence of pus is a definitive sign of active infection and progression.
- Radiographic changes: Progressive marginal bone loss beyond the expected initial remodeling phase is a critical diagnostic criterion.
Diagnostic Protocol
- Establish a baseline: Record probing depths, BOP, and radiographs at the time of final restoration placement.
- Monitor at each recall: Compare probing depths and radiographs to baseline values.
- Use consistent probing pressure: Excessive force can cause false positives for bleeding or damage tissues.
- Integrate adjunctive tools: Peri‑implant sulcular fluid analysis for inflammatory markers (e.g., IL‑1β) and bacterial DNA testing can help identify high‑risk cases before radiographic changes appear.
The sooner clinicians recognize subtle signs — such as slight increases in probing depth combined with localized bleeding — the greater the likelihood of halting disease progression without invasive treatment.
Current Evidence‑Based Treatment Strategies
Management depends on disease severity and combines mechanical, chemical, and surgical modalities.
Non‑Surgical Approaches
- Mechanical debridement with titanium or carbon fiber instruments to minimize surface damage.
- Adjunctive antimicrobials, including chlorhexidine irrigation or local minocycline application⁹.
- Air‑powder abrasive devices using glycine or erythritol powders for biofilm disruption¹⁰.
Non‑surgical therapy is most effective in early disease stages but often insufficient in advanced peri‑implantitis.
Surgical Approaches
When non‑surgical measures fail, surgery may be indicated to access contaminated implant surfaces, reduce pockets, and regenerate lost bone:
- Open‑flap debridement for thorough cleaning.
- Resective surgery to reshape peri‑implant bone defects.
- Regenerative surgery using bone grafts and membranes to restore bone support¹¹.
Novel and Adjunctive Therapies
Emerging studies highlight promising options:
- Er:YAG and diode lasers for decontamination.
- Photodynamic therapy (PDT) to enhance bacterial reduction.
- Probiotic therapy for microbiome modulation.
While early results are encouraging, long‑term comparative studies are still limited.
Strengthening Prevention and Maintenance Protocols
While treatment options have evolved, prevention remains the cornerstone of implant longevity. A structured maintenance program reduces the risk of peri‑implantitis and supports the stability of peri‑implant tissues.
Professional Maintenance Intervals
- Every 3–4 months for patients with previous periodontitis, systemic risk factors, or reduced dexterity.
- Every 6 months for low‑risk patients with excellent home care.
These visits should include mechanical plaque removal with non‑metallic scalers, low‑abrasive polishing, and periodic reinforcement of oral hygiene techniques.
Patient‑Directed Strategies
- Effective plaque control: Twice‑daily brushing with a soft brush or powered toothbrush and interdental cleaning with floss, interdental brushes, or water flossers.
- Antimicrobial rinses: Short‑term chlorhexidine mouthwash during inflammation flare‑ups can reduce bacterial load.
- Avoidance of risk behaviors: Smoking cessation and strict glycemic control in diabetic patients are strongly linked to lower peri‑implant disease rates.
Customizing Maintenance for High‑Risk Patients
For patients with systemic disease, history of periodontitis, or complex implant restorations (e.g., full‑arch prostheses), maintenance should include:
- More frequent professional debridement.
- Regular microbial testing for high‑risk pathogens.
- Reinforcement of home‑care protocols at every visit.
Patient Education
The clinician’s role extends beyond clinical intervention. Educating patients about the signs of peri‑implant inflammation — such as redness, swelling, tenderness, or bleeding — empowers them to seek help before advanced bone loss occurs.
Key Takeaway for the Modern Implant Practice
Peri‑implantitis is a multifactorial disease that requires vigilance, patient education, and a tailored treatment approach. By identifying high‑risk patients early, applying evidence‑based interventions, and committing to robust maintenance protocols, clinicians can significantly improve implant survival rates and patient satisfaction.
References
- Berglundh T, et al. J Clin Periodontol. 2018;45(Suppl 20):S286‑S291.
- Schwarz F, et al. Periodontol 2000. 2019;79(1):211‑223.
- Renvert S, et al. J Clin Periodontol. 2018;45(5):568‑581.
- Heitz‑Mayfield LJA, et al. J Clin Periodontol. 2020;47(Suppl 22):S256‑S261.
- Levin L, et al. Clin Implant Dent Relat Res. 2011;13(4):349‑353.
- Ferreira SD, et al. J Clin Periodontol. 2006;33(12):929‑934.
- Monje A, et al. J Dent Res. 2016;95(4):372‑379.
- Sanz M, et al. Clin Oral Implants Res. 2012;23(Suppl 6):108‑110.
- Renvert S, Polyzois I. J Clin Periodontol. 2015;42(Suppl 16):S152‑S163.
- Flemmig TF, et al. J Clin Periodontol. 2012;39(9):889‑897.
- Roccuzzo M, et al. Clin Oral Implants Res. 2011;22(7):682‑690.
- Lang NP, et al. J Clin Periodontol. 2000;27(4):240‑246.